| « Cleaning Your Vehicle's Throttle Plate is Easier Than You Thought | Is A.I. (Artificial Intelligence) Coming for Your Job? » |
Pro-Vaxxer Trump IS Lining Up to Be The Vaccine "Colt Seavers"
The Beginning
When reading this article, keep in mind that the influenza pandemic documents, and Executive Order 13887 of September 19, 2019 I'm referencing -- appear long before there was any talk of COVID-19 anywhere. However, the groundwork was being set to ramp up the mass production of vaccines "just in case of a pandemic."
From what I've seen and been able to find recently, things are not looking good for those who assumed that Trump was going to sweep in to DC and "fix" things.
There are documents from 2019, detailing the need for a new vaccine, "just in case" of a flu pandemic. Dr. Fauci warned about this in 2017.
Dr. Anthony Fauci, the U.S. government’s top infectious disease specialist, warned in early 2017 that a “surprise outbreak” would occur during the Trump administration, and he said that more needed to be done to prepare for a pandemic.
“There is no question that there will be a challenge to the coming administration in the arena of infectious diseases,” he said in a speech titled “Pandemic Preparedness in the Next Administration” at Georgetown University Medical Center. He delivered it just days before Trump was inaugurated on Jan. 20, 2017.
How could Dr. Anthony Fauci possibly "know" that there, to quote him, "will be" a pandemic during the next administration.
Enough people were worried about infectious diseases to convince the The Council of Economic Advisors to generate a report on the subject.
The document titled "Mitigating the Impact of Pandemic Influenza through Vaccine Innovation" which outlines the need for mRNA and other new vaccine technology can be found here (on the official ".gov" website). It lists mRNA as one of the emerging technologies that might be used to drastically decrease the time required to produce a new vaccine.
Here are a few excerpts from the document "Mitigating the Impact of Pandemic Influenza through Vaccine Innovation" from The Council of Economic Advisors September 2019.
Executive Summary
September 2019This report estimates the potentially large health and economic losses in the United States associated with influenza pandemics and discusses why the most commonly used vaccine production technologies are unlikely to mitigate these losses. We estimate the value of new vaccine technologies that would make vaccines available more quickly and likely improve their effectiveness in moderating the risks of pandemics. We discuss why private market incentives may be insufficient to develop new vaccine technologies or promote the uptake of existing, faster but more expensive technologies, despite their large expected value to society. And we argue that increased utilization of, and investment in, these new technologies—along with public-private partnerships, to spur innovation—may be valuable to decrease the impact of both pandemic and seasonal influenza.
...
The Council of Economic Advisors (CEA) finds that in a pandemic year, depending on the transmission efficiency and virulence of the particular pandemic virus, the economic damage would range from $413 billion to $3.79 trillion. Fatalities in the most serious scenario would exceed half a million people in the United States. Millions more would be sick, with between approximately 670,000 to 4.3 million requiring hospitalization. In a severe pandemic, healthy people might avoid work and normal social interactions in an attempt to avert illness by limiting contact with sick persons. By incapacitating a large fraction of the population, including individuals who work in critical infrastructure and defense sectors, pandemic influenza could threaten U.S. national security.Large-scale, immediate immunization is the most effective way to control the spread of influenza, but the predominant, currently licensed, vaccine manufacturing technology would not provide sufficient doses rapidly enough to mitigate a pandemic. Current influenza vaccine production focuses on providing vaccines for the seasonal flu and primarily relies on growing viruses in chicken eggs. Egg-based production can take six months or more to deliver substantial amounts of vaccines after a pathogenic, influenza virus is identified—too slowly to stave off the rapid spread of infections if an unexpected and highly contagious pandemic virus emerges.
To summarize the push for this new vaccine technology is that; in the event of a pandemic, it will help to minimize death and illness, minimize the economic impact, but most importantly illness within the general population is now considered a matter of national security. All existing "currently licensed" vaccine manufacturing tech is too slow. We need something new that can be produced faster.
This next part truly amazed me, but is illustrative of exactly what we saw during the "pandemic."
Improving the speed of vaccine production and vaccine efficacy are both important goals to mitigate pandemic risks and may also decrease the costs of seasonal influenza. Our analysis shows that innovation to increase the speed of vaccine production is key. Improving vaccine efficacy alone will be of little value in a pandemic if, as is the case with current egg-based production, the vaccine only becomes available after a large number of infections have occurred. Improving efficacy only yields value after greater speed has been achieved.
Their analysis shows that getting the vaccine manufactured, distributed and injected into as many people as quickly as possible -- is more important than vaccine effectiveness? Effectiveness is of little concern. While testing for effectiveness, the process also determines if there are unwanted side effects. Unverified effectiveness will result in unverified/unknown safety issues as well. In other words, if vaccine effectiveness is not a priority -- neither can vaccine safety be a priority. "Safe and effective" goes hand-in-hand with any injectable. You can't have one without the other.
And yet, someone, within the Trump administration, looked at this and thought, "Yeah, this is what we need here. More injectables that 'might probably' work."
Another production process, self-amplifying mRNA (SAM) vaccine manufacturing, which is patented but does not yet have an FDA-approved product, could shorten the vaccine manufacturing timeline even further. The SAM [mRNA] vaccine has been shown to be effective in mice (Hekele et al. 2013). Per interviews with government experts on influenza vaccines, both recombinant and SAM vaccines hold great promise for substantially shortening the vaccine manufacturing timeline and may provide the flexibility to engineer what would be a significant advance in the fight against influenza—a “universal” influenza vaccine.
...
Given the underprovision of pandemic risk mitigation by the private sector, the public sector [government] has a role in stimulating the development of, and demand for, newer vaccine technologies that are better able to provide pandemic preparedness. Public-private partnerships that stimulate such innovation may enhance welfare.
This publication by The Council of Economic Advisors concludes that the government, "has a role in, stimulating the development and demand for," newer vaccine technologies. What that means is that the government is going to pay private industry to develop "newer vaccine technologies" under the direction of the the government.
However, there is one component never mentioned in this document. There is no mention of liability. The reason that the private sector hasn't tried to drastically expand newer vaccine technology is because they have hit many dead ends. The mRNA technology mentioned in this publication has a very bad, and very well documented track record which is detailed in this NIH document from April 24, 2019.
I'll go so far as to say that it mRNA was/is not promising. This is not my opinion, this is well documented. Nobody has made it work, without serious complications to the test subjects. With these types of setbacks, private industry has been understandably reluctant to move forward with these newer vaccine technologies.
2019 and Beyond
Amazingly an executive order was drawn up -- and signed into law by September 19, 2019? Assuming that the first publication I cited was delivered on September 1st, 2019, the Trump administration had a whole (19) days to review the report, approve action on based on the report, draft an executive order, have it reviewed -- and signed by the 19th! Does anyone really believe that our government is this efficient? Or, was it more likely that these measures were drafted ahead of time?
Here's a link to Executive Order 13887 of September 19, 2019. You can also download a PDF version from there as well.
The entire beginning of the executive order (EO) is nearly a copy and paste of the document above. I'm going to list the parts that seem the most relevant.
Executive Order 13887 of September 19, 2019
Modernizing Influenza Vaccines in the United States to Promote National Security and Public Health
By the authority vested in me as President by the Constitution and the laws of the United States of America, including section 301 of title 3, United States Code, it is hereby ordered as follows:
Section 1. Findings. a) Influenza viruses are constantly changing as they circulate globally in humans and animals. Relatively minor changes in these viruses cause annual seasonal influenza outbreaks, which result in millions of illnesses, hundreds of thousands of hospitalizations, and tens of thousands of deaths each year in the United States. Periodically, new influenza A viruses emerge from animals, including birds and pigs, that can spread efficiently and have sustained transmission among humans. This situation is called an influenza pandemic (pandemic). Unlike seasonal influenza, a pandemic has the potential to spread rapidly around the globe, infect higher numbers of people, and cause high rates of illness and death in populations that lack prior immunity. While it is not possible to predict when or how frequently a pandemic may occur, there have been 4 pandemics in the last 100 years. The most devastating pandemic occurred in 1918–1919 and is estimated to have killed more than 50 million people worldwide, including 675,000 Americans.
...
(e) The seasonal influenza vaccine market rewards manufacturers that deliver vaccines in time for the influenza season, without consideration of the speed or scale of these manufacturers’ production processes. This approach is insufficient to meet the response needs in the event of a pandemic, which can emerge rapidly and with little warning. Because the market does not sufficiently reward speed, and because a pandemic has the potential to overwhelm or compromise essential government functions, including defense and homeland security, the Government must take action to promote faster and more scalable manufacturing platforms.
What part (e) means is that the Government must take over the vaccine industry.
Sec. 2. Policy. It is the policy of the United States to modernize the domestic influenza vaccine enterprise to be highly responsive, flexible, scalable, and more effective at preventing the spread of influenza viruses. This is a public health and national security priority, as influenza has the potential to significantly harm the United States and our interests, including through largescale illness and death, disruption to military operations, and damage to the economy. This order directs actions to reduce the United States’ reliance on egg-based influenza vaccine production; to expand domestic capacity of alternative methods that allow more agile and rapid responses to emerging influenza viruses; to advance the development of new, broadly protective vaccine candidates that provide more effective and longer lasting immunities; and to support the promotion of increased influenza vaccine immunization across recommended populations.
Sec. 3. National Influenza Vaccine Task Force.
(a) There is hereby established a National Influenza Vaccine Task Force (Task Force). The Task Force shall identify actions to achieve the objectives identified in section 2 of this order and monitor and report on the implementation and results of those actions. The Task Force shall be co-chaired by the Secretary of Defense and the Secretary of Health and Human Services, or their designees.
(b) In addition to the Co-Chairs, the Task Force shall consist of a senior official from the following executive branch departments, agencies, and offices:
(i) the Department of Defense (DOD);
(ii) the Department of Justice; [DOJ]
(iii) the Department of Agriculture; [USDA]
(iv) the Department of Veterans Affairs (VA);
(v) the Department of Homeland Security; [DHS]
(vi) the United States Food and Drug Administration; [FDA]
(vii) the Centers for Disease Control and Prevention; [CDC]
(viii) the National Institutes of Health (NIH);
(ix) the Centers for Medicare and Medicaid Services (CMS); and
(x) the Biomedical Advanced Research and Development Authority (BARDA).(c) The Co-Chairs may jointly invite additional Federal Government representatives, with the consent of the applicable executive department, agency, or office head, to attend meetings of the Task Force or to become members of the Task Force, as appropriate.
(d) The staffs of the Department of State, the Office of Management and Budget (OMB), the National Security Council, the Council of Economic Advisers, the Domestic Policy Council, the National Economic Council, and the Office of Science and Technology Policy (OSTP) may attend and participate in any Task Force meetings or discussions.
(e) The Task Force may consult with State, local, tribal, and territorial government officials and private sector representatives, as appropriate and consistent with applicable law.
(f) Within 120 days of the date of this order, the Task Force shall submit a report to the President, through the Assistant to the President for National Security Affairs, the Assistant to the President for Domestic Policy, the Director of the Office of Management and Budget, and the Director of the Office of Science and Technology Policy. The report shall include:
(i) a 5-year national plan (Plan) to promote the use of more agile and scalable vaccine manufacturing technologies and to accelerate development of vaccines that protect against many or all influenza viruses;
(ii) recommendations for encouraging non-profit, academic, and private sector influenza vaccine innovation; and
(iii) recommendations for increasing influenza vaccination among the populations recommended by the CDC and for improving public understanding of influenza risk and informed influenza vaccine decision-making.
(g) Not later than June 1 of each of the 5 years following submission of the report described in subsection (f) of this section, the Task Force shall submit an update on implementation of the Plan and, as appropriate, new recommendations for achieving the policy objectives set forth in section 2 of this order.
I know this is a long article, but bear with me here. There are few more important dates to cover.
The EO states that there's supposed to be a report generated within 120 days of its issuance.
September 19, 2019 + (120 days) = Friday, January 17, 2020.
Keep this in mind when you see these pandemic response timeline straight from BARDA's website. Everything lines up precisely. Are the people involved in pandemic preparedness literal fortune tellers?
On Friday, January 10, 2020 the Chinese government publicly shares the gene sequence of the novel coronavirus. The following Friday, January 17, 2020 is the deadline for delivery of the report from the National Influenza Vaccine Task Force. And on the following Monday January 20, 2020, (just 10-days later) the US has it's first confirmed case of COVID-19.
The response to COVID-19/SARS-CoV-2 was initiated before there was an actual pandemic declaration. Which might seem like a good thing, we were prepared right?
That is, until you realize that this series of documented events lines up precisely -- within days, to make all of these agencies and public-private partnerships come together.
The White House didn't form their Coronavirus Task Force until January 29, 2019. It's possible to assume that Trump was unaware of the steps leading up to these events. While Trump's ignorance is possible, it's not very probable.
At this point -- there is absolutely no way that Trump isn't aware of the pandemic response outcome.
In any event, everybody needs to start preparing for things going forward.
I have no reason to assume that the pandemic racket is going away under Trump. The pandemic racket was formed while Trump was in office -- and signed off on by Trump himself.
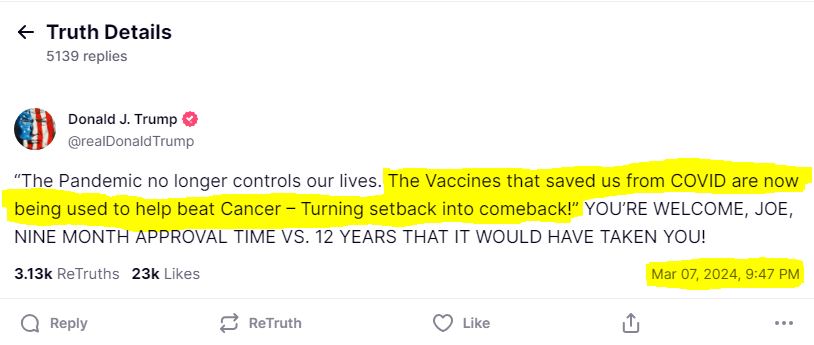
Then Trump said this during the State of The Union Show on March 7, 2024.
Keep in mind that BARDA is the medical counterpart to DARPA. Hence Medical Countermeasures.
What do you think?
Please leave a comment, like it or hate it... You DO NOT need to register to leave a comment. Email addresses are NOT used. Just make one up "someone@somehost.com"